
|
 |
Research > Science
Highlights > Pumping Iron on the Seafloor
Pumping Iron on the Seafloor
Somewhere on the vast seafloor that covers 70 percent of our
planet lies a small rock. On that rock is a microscopic crevice,
maybe 10 microns (0.0004 inches) deep and 20 microns wide. And
in that crevice is a thriving community of microbes, for whom
that nook is as cozy and bountiful as Grandma’s house on Thanksgiving.
 |
 |
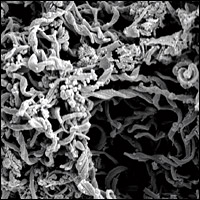 |
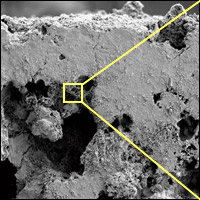
Scientists at WHOI deployed blocks of rock samples containing iron sulfide on the seafloor and retrieved them two months later. The scanning electron micrograph (left) shows a close-up view of pores on the surface of a rock sample. The further magnified image (right) shows huge accumulations of iron oxide material metabolized by iron-oxidizing bacteria that flourish within the pore spaces. (Photo by Katrina Edwards)
|
That tiny sheltered cove contains all the ingredients
the microbes need. They convert carbon dioxide from seawater into organic matter, and extract a little oxygen or nitrogen to breathe. From the surrounding volcanic rock they extract iron.
The microbes steal electrons from iron atoms—changing them from ferrous (Fe+2) to ferric (Fe+3). They harness the energy produced by this chemical reaction to grow and multiply. In the sunless depths, where photosynthesis isn’t an option, these iron-oxidizing
microorganisms are making a good living.
Over the past few years, said WHOI geochemist
Katrina Edwards “we have found large numbers of previously unidentified, iron-oxidizing microbes, living
directly off the minerals in seafloor rocks.”
Using the submersible Alvin, Edwards and colleagues
in the Marine Chemistry and Geochemistry Department placed microbe-free
samples of natural iron-rich seafloor rock back on the seafloor.
Retrieved several months later, the samples had thick orange
coatings of oxidized iron. In tiny pores and pits on the sample
surfaces, microbes grew and flourished.
In 2003, near Loihi, an active, submerged volcano off the Big Island of Hawaii, scientists set up an
observatory to study iron bacteria at a site where they are unusually prolific. The seafloor at Loihi is carpeted with ocher-red bacterial mats, made by
microbial communities living off iron in the rock and in hydrothermal fluids venting from the seafloor. “They are really spectacular,” said WHOI geochemist Dan Rogers.
“People have known about the existence of these microbes for decades,” said WHOI geochemist Wolfgang Bach. “But no one thought they would be so abundant and ubiquitous.”
 |
| A reddish-orange
iron oxide material coats the seafloor on Loihi Seamount,
an active underwater volcano off Hawaii. The material,
similar to rust, is made by an abundance of microbes oxidizing
iron in seafloor rocks to live and grow. (Photo by Terry
Kirby, University of Hawaii) |
If the oceans do contain large, widespread populations
of these tiny overlooked microbes, it could revolutionize our thinking about colossal mysteries: What regulates the ocean’s chemistry and the planet’s climate? How did life evolve on Earth and perhaps on other planets?
Rocks on the seafloor and even below it, have potentially infinite
niches, where the chemistry is just right for iron-oxidizing
microbes. Edwards and Bach analyzed rock samples drilled from
the exposed volcanic rock that spreads out on both sides of
volcanic seafloor mountains. They found that older rocks were
depleted of Fe+2 and full of Fe+3—exactly
what iron-oxidizing microbes use up and leave behind. The flanks
of mid-ocean ridges encircling the globe could represent millions
of square miles of fertile territory for innumerable microbial
hordes.
Such a massive microbial community would continually
extract huge amounts of carbon dioxide, an important greenhouse gas, from seawater, and microscopically
mediate large changes in ocean chemistry over geologic time. The drawdown of carbon dioxide into solid rock could revise our understanding of how carbon cycles through the planetary system, giving the microbes an important role in the evolution of Earth’s climate.
Plentiful populations of iron-oxidizing microbes might also form the base of a food chain supporting seafloor ecosystems. They might even have been pioneering
life forms on early Earth—more than
2.7 billion years ago, when the oceans were rich with volcanic rock, but before plants appeared to supply the planet’s atmosphere with oxygen. Similar microbes could have thrived, or may still thrive, in other iron-rich, oxygen-poor locales—such as Mars, for example.
The research “requires you to be a little bit of a biologist, chemist, and geologist,” Rogers said. He and others in Edwards’ lab are working to identify the microbes, calculate their numbers, and decipher their biochemical machinery and metabolic capabilities. With Mitch Sogin at Marine Biological Laboratory in Woods Hole, Edwards is pursuing genomic studies to help determine these microbes’ place in evolutionary
history.
Bach, meanwhile, is using new technology, a gallium ion beam, to cut 100-nanometer-thick rock samples and learn how microbes affect the rocks’ microscopic texture and composition. A reddish-orange iron oxide material coats the seafloor on Loihi Seamount, an active underwater volcano off Hawaii. The material, similar to rust, is made by an abundance of microbes oxidizing iron in seafloor rocks to live and grow.
With such knowledge, scientists will be able to distinguish
textures caused by microbial activity from those caused by abiotic
oxidizing processes such as rusting. With lessons learned from
textural, isotopic, molecular, and other studies, Bach said,
“we strive to able to look at a 70-million-year-old rock—or
a Martian rock—and say with certainty, ‘microbes were here.’
”
—Lonny Lippsett (llippsett@whoi.edu)
|
|
