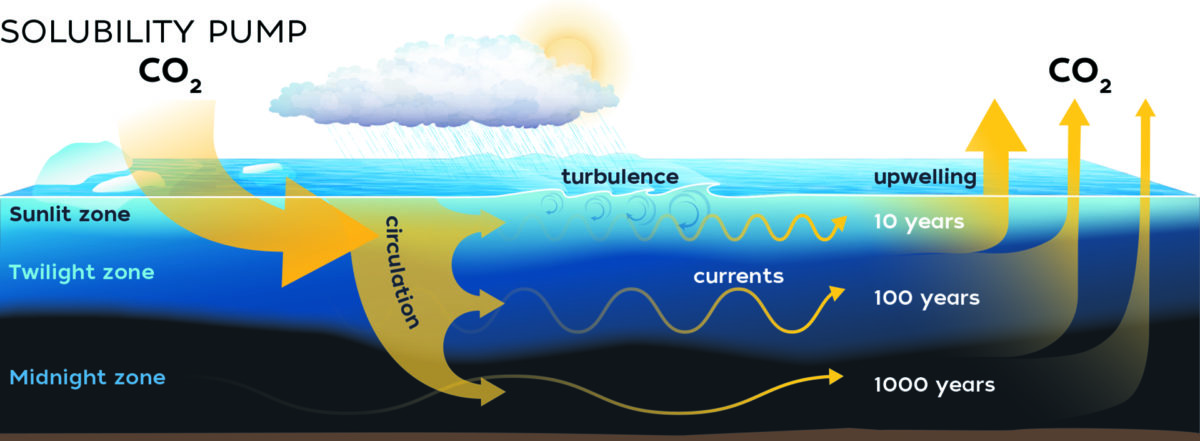
Solubility Pump: Many different gases dissolve in seawater including oxygen, nitrogen and carbon dioxide. Solubility of CO2 is strongly dependent on temperature, among other factors. The colder the water, the more CO2 it can hold. Indeed, most of the CO2 from atmospheric sources is dissolved in cold water of the high latitudes (particularly so in the north Atlantic) Each year, the global ocean absorbs about 25% of all carbon emitted into the atmosphere from human activities. 19 Alarmingly, the efficiency of seawater to dissolve CO2 is expected to decrease as the ocean continues to warm. In polar regions, where solubility of CO2 is high, sea ice formation leaves the surrounding waters very salty and dense. The dense water then sinks, generating a thermohaline’ circulation that continuously moves water equatorward in the deep ocean (known as the global conveyor belt) Therefore, the deep sea stores vast quantities of dissolved inorganic carbon (DIC) on long timescales. T his process of atmospheric CO2 dissolving and reacting in the sea surface to form DIC then being transported into the deep sea by wind and thermohaline circulation is known as the solubility pump.’ It is important to note that CO2 also outgasses back into the atmosphere (just as a carbonated beverage becomes flat after opening the container), therefore the balance of carbon between ocean and atmosphere is the important factor in relation to how much GHG is in the atmosphere at any given time. (Illustration by Natalie Renier, © Woods Hole Oceanographic Institution)
Image and Visual Licensing
WHOI copyright digital assets (stills and video) contained on this website can be licensed for non-commercial use upon request and approval. Please contact WHOI Digital Assets at images@whoi.edu or (508) 289-2647.