Sunlight and the fate of oil at sea
Danielle Haas Freeman draws on the language of chemistry to solve an oil spill puzzle
Estimated reading time: 6 minutes
In April of 2010, the Deepwater Horizon oil rig, located off the coast of Louisiana, experienced a catastrophic fire. Eleven people working on the rig died in the explosion. Unseen, about a mile below this human tragedy, the Macondo oil well connected to the Deepwater Horizon rig (by a long, long pipe) ruptured, releasing barrel upon barrel of oil and natural gas into the Gulf of Mexico. The location of the oil release, a mile deep, underwater, at high pressure, meant that emergency responders didn’t have a preexisting blueprint for how to stop the spill. So, for 87 days, the ill-fated, aptly-named Macondo oil and gas continued to flow into the Gulf, with about half of it staying underwater (the “plume”) and half of it floating up to the sea surface and forming miles of oil slicks. By the time the well was plugged, 4.2 million barrels of oil had been released (McNutt et al., 2012).
I was in high school when Deepwater Horizon happened. In the New York City apartment I grew up in, watching the 6:30 pm national news was a ritual that my father did not allow anyone or anything to interrupt. So, for most evenings in the spring and summer of 2010, we watched the news updates on what the anchor called “the BP oil spill,” in reference to the company that owned the well. I remember the underwater video footage of dark oil flooding out of the well—some of which I now know was likely captured by WHOI scientists and engineers—and I remember an overwhelming sense of powerlessness.

MIT-WHOI Joint Program student Danielle Haas Freeman. (Photo by Daniel Hentz, © Woods Hole Oceanographic Institution)
At that point in my education, science meant homework. It meant memorizing the definitions of protons and neutrons and classes that were structured around endless projector transparencies full of notes. Science did not mean empowerment. It did not mean understanding the inner workings of the world around me in more than an abstract way. And, it did not mean uncovering knowledge that could help us prevent or mitigate disasters like Deepwater Horizon.
Luckily for me, I dedicated enough time to the projector transparencies to develop a lukewarm interest in science during college, where I took classes in chemistry and environmental science. These classes, it turned out, completely changed my perspective on the value of science and its capacity to confront problems like Deepwater Horizon. Chemistry, I learned, was an amazingly powerful language that underwrote all of our physical world, a language scientists could translate to reveal new knowledge about earth’s history, climate, and present pollution events like Deepwater Horizon. Upon practicing the grammar and vocabulary of this new language, there was no going back. The feeling of empowerment generated by those classes brought me to Dr. Collin Ward’s lab at WHOI, where I now study what happens to oil after it is spilled at sea (its “environmental fate”) during disasters like Deepwater Horizon.
Oil fate and sunlight
What happened to all the oil spilled during Deepwater Horizon? Twelve years later, scientists are still trying to answer this question. Some pieces of the puzzle are well recognized. For example, we know that some of the oil evaporated off of the sea surface into the atmosphere. And we know that a lot of the underwater plume oil was eaten by microbes (biodegradation).
A recent project I’ve worked on gives a size and shape to another piece of the puzzle: the transformation of crude oil by sunlight-initiated chemical reactions into new compounds. These compounds dissolved in seawater during the oil spill, acting as a removal process from those floating surface oil slicks.
How and why would this happen? You might know that oil and water don’t mix. This is why floating oil slicks form on the sea surface in the first place. The rainbow oil sheens you might see in a puddle near a gas station or on a city street are examples of this phenomenon. But sunlight exposure can cause chemical reactions that change the qualities of oil. Some of the new compounds generated by this sunlight-driven chemistry (“photochemistry”) do mix with water easily, like sugar dissolving into hot tea. We call this phenomenon “photo-dissolution” (“photo” means “light”).
Scientists have known for a long time that this process can affect oil, but no one knew how much it could affect oil. That’s where our project comes in. We set out to quantify how fast oil photo-dissolution happens, and to determine the impact this process likely had on Deepwater Horizon, and could have on future spills.
To do this, we couldn’t just shine any old light on our oil sample for any amount of time and throw it in water to see how much dissolved. We had to study how the oil was changed by different colors of light in the ultraviolet (high-energy light invisible to the human eye) and visible (lower-energy light that makes up our rainbow, from violet to red) range for different time periods of light exposure. We used light-emitting diode (LED)-based photo-reactors that were custom-built in our lab to irradiate the oil under these different conditions. Then, we exposed the oil to seawater and measured how much it dissolved. We took our data from the lab and used the results to calculate the fraction of oil which likely dissolved into seawater via this process during Deepwater Horizon. We also plugged the data into a bunch of hypothetical spill scenarios that could happen at different times of year, at different latitudes, etc., to explore how important this process might be in future spills that happen under different conditions.
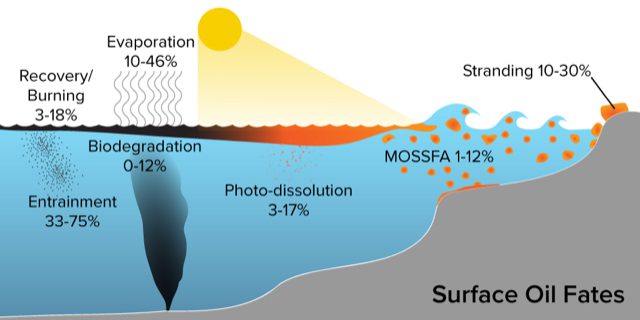
Surface oil floating on the Gulf of Mexico after the Deepwater Horizon spill was subject to many environmental processes, including not only photo-dissolution but also evaporation, entrainment, and more (shown in the diagram). Percentages show the fraction of oil impacted by each process. Note that not all of the processes are independent of each other (i.e., some oil that is entrained may also be subsequently impacted by photo-dissolution or biodegradation) so the percentages do not add up to 100 percent. (Illustration by Natalie Renier, © Woods Hole Oceanographic Institution)
What did we learn?
Our experimental results showed that photo-dissolution happens fast—fast enough that, implied by our experimental results, it could have removed almost 10% of the surface oil during the Deepwater Horizon spill. While 10% might seem small, keep in mind that the oil fate puzzle is split up into many small puzzle pieces, and 10% is similar in magnitude to the fractions of surface oil removed by other well-recognized fate processes, such as the stranding of oil along the beaches of the Gulf coast.
We also found out that sunlight-driven reactions can have implications beyond Deepwater Horizon—for example, we found that these reactions could be important in the event of an ice spill in the Arctic, a region with heightened risk of oil spill because of less sea ice and higher ship traffic.
Why does it matter?
We don’t know yet whether the net effect of oil photo-dissolution is good or bad after a spill. For example, if photo-dissolution is removing a substantial amount of oil from the sea surface, that could mean that less oil is making it to sensitive coastal areas, which is a good outcome. On the other hand, there isn’t a lot of data on the toxicity of these compounds to marine life, so we don’t know what the risks are. Determining this will be an important next step for research on this topic.
Our hope is that incorporation of the photo-dissolution puzzle piece into future experimental research, along with oil spill modeling, will give spill responders improved spill forecasts so cleanups are more effective and damage assessments are more accurate. Thinking back to the feeling of empowerment that I developed during college environmental chemistry classes, it’s exactly this outcome that I am most excited about—the knowledge that our science can move us just a little closer to understanding environmental disasters and protecting humans and ecosystems when they occur.
Funding for this research was received from an NSF-GRFP grant and Fisheries and Oceans Canada’s Multi-Partner Research Initiative. This story was adapted from the blog post: https://www2.whoi.edu/staff/dfreeman/dissolving-oil-in-a-sunlit-sea/. A similar adaptation appeared in Fisheries and Oceans Canada’s Multi-Partner Research Initiative Newsletter Spring/Summer 2022, Issue #4